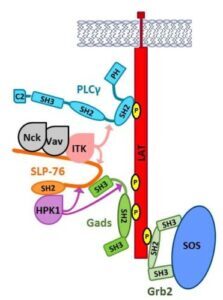
T cells and mast cells share a high sensitivity to antigen. In both cell types, antigen recognition triggers the tyrosine phosphorylation of two key adaptor proteins, LAT and SLP-76. A third adaptor, Gads, bridges the recruitment of SLP-76 to LAT, and together these adaptors nucleate the formation of large signaling complexes that transmit antigen receptor signals into the cell.
Our group studies the regulatory mechanisms that govern adaptor protein signaling pathways. We characterized the role of SLP-76 in recruiting and activating specific kinases that regulate antigen receptor responsiveness. We identified and characterized new phosphorylation sites on SLP-76 and Gads that regulate their signaling functions. We are studying cooperative interactions within the LAT-nucleated signaling complex, and the role of SH2 domain dimerization in mediating cooperativity. Our research combines biochemical, biophysical and genetic approaches to reach an in depth understanding of the mechanisms underlying the sensitivity and selectivity of antigen responsiveness.